Interplay between lymphocyte subpopulation, inflammatory cytokines and their correlation with oxidative stress parameters in COVID-19
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Accepted: 13 December 2022
Authors
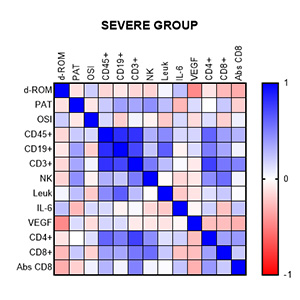
Our objective was to investigate the inflammatory and oxidative stress markers in patients with moderate and severe form of coronavirus disease 2019 (COVID-19). In addition, we show the correlation between changes in lymphocyte subsets and markers of oxidative stress as a tool for patient classification. Interleukin-6 (IL-6) and VEGF were analyzed by utilizing a High Sensitivity Evidence Investigator™ Biochip Array technology. The total antioxidant capacity (PAT) and the free radical concentrations (d-ROM) were measured in serum utilizing analytical photometric system FRAS5. Peripheral blood was used to determine CD45 + mononuclear, B, T, and NK cells using a multi-parameter flow cytometric immunophenotypic test. Statistically significant differences in IL-6 and VEGF levels were observed between the two patient groups. Decreased values of the absolute number of lymphocytes and their CD4 + and CD8 + positive T cells, NK cells, and CD8 were obtained. In the moderate group, good correlations were found between IL-6 and VEGF and NK cells (r=0.6973, P<0.05; for IL-6 and r=0.6498, P<0, for VEGF. 05). Cytokines were correlated with CD45+ (r=0.5610, P<0.05; for IL-6 and r=0.5462, P<0.05 for VEGF). The oxidative stress index can be used as a cheaper alternative and as a triage tool between severe and moderate illnesses, after showing good correlation with more expensive patient classification analysis.
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






